|
Category |
Licensing Procedure |
|
Responsible Agency |
Pharmaceutical and Regulatory Affairs Address: 10-16 Grenada Way Kingston 5 Phone: 876-633-8172 Email: |
|
Legal base of the Procedure |
The Foods and Drugs Act |
| Fee | $ 5,000 JMD |
Required Information
|
No. |
Type of information |
Note |
|
1 |
Application Form, supporting documents |
Process Steps
|
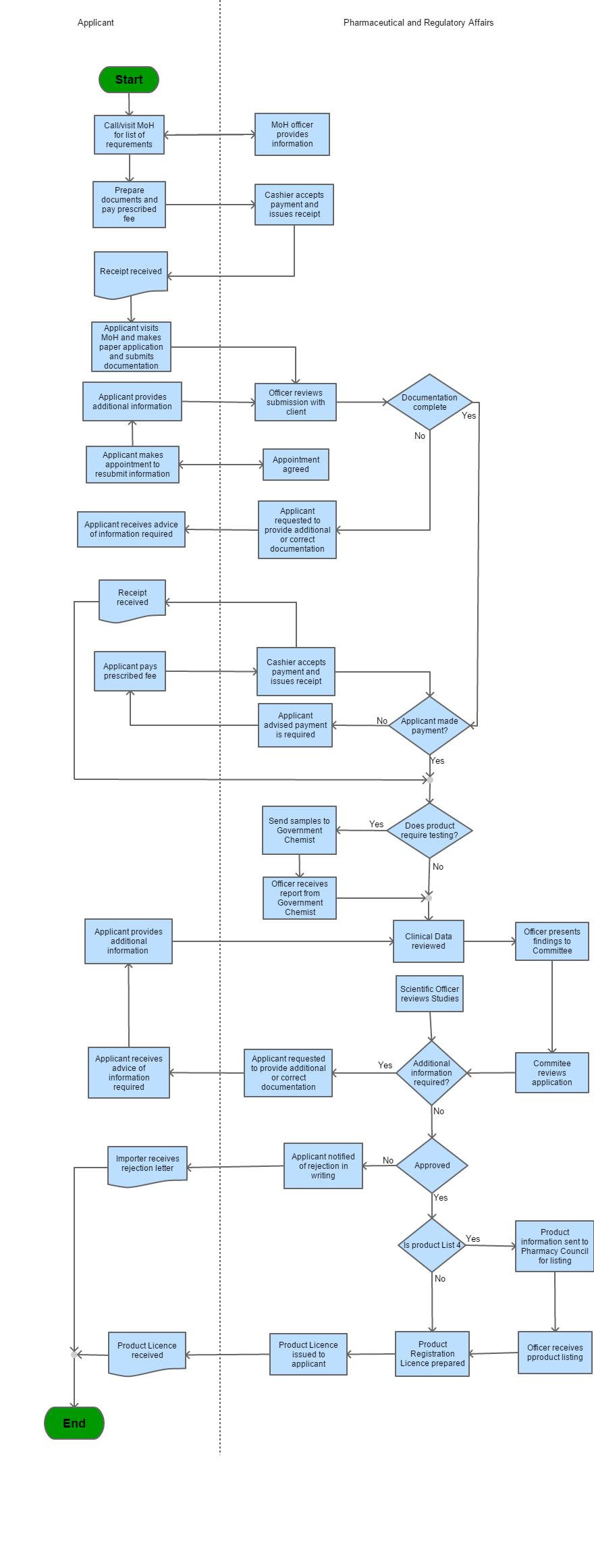
Step 1 |
Applicant visits/ calls MOH for the List of Requirements and information is provided by MOH officer |
|
|
Step 2 |
Applicant prepares the document and pays prescribed fee | |
|
Step 3 |
The cashier accepts payment and issues the receipt |
|
|
Step 4 |
The receipt is received |
|
|
Step 5 |
Applicant visit MOH and makes the paper application and submits documentation | |
| Step 6 | Officer reviews submission with client | |
| Step 7 |
a) If documentation is deemed incomplete then: i) The cashier accepts payment and issue required b) The payment has been made require |
|
| Step 8 |
a) If payment has not been made then: i) The applicant pays the prescribed fee ii) The payment is received and receipt is issued b) If payment has been made receipt will be issued and the product requires testing |
|
| Step 9 |
If the product requires testing then: i) Samples are sent to the Government Chemist ii) Officers receives report from Government Chemist iii) Clinical Data received If the product does not require testing then clinical data is received
|
|
| Step 10 | Officer then presents findings to the Committee | |
| Step 11 | The application is reviewed | |
| Step 12 |
If additional information is received then: i) Applicant is requested to provide additional information or correct documentation ii) Applicant receives advice about required information and information is provided by the applicant iii) Applicants will follow Step 9 after additional information is given by the applicant If no additional information is required then the product is considered for approval |
|
| Step 13 |
If the product is a List 4 then: i) Product Information sent to Pharmacy Council for listing ii) Officer receives product listing iii) Product Registration is prepared iv) Product License is issued to the applicant v) Product License is received If the product is not under list 4 then: i) Product Registration is prepared ii) Product License is issued to the applicant iii) Product License is received |
| # | Title | Description | Issued By | File |
|---|---|---|---|---|
| 1 | Registration of New Drugs (Ministry of Health) | Registration of New Drugs (Foods and Drugs Act 1964) | Ministry of Health & Wellness |
| # | Name | Description | Measure Type | Agency | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|---|
| 1 | License for the Registration of Pharmaceutical Products | A person shall not sell, manufacture, import or distribute drugs which are not registered with the Ministry of Health and a fee of $25.00 has been paid in respect of such registration. | Licensing Requirement | Ministry of Health & Wellness | The Minister of Health has the discretion exempt any person from these requirements. | The Food and Drugs Act | 9999-09-09 | Good |
Please share your feedback below and help us improve our content.
