|
Category |
Import Permit |
|
Responsible Agency |
Pharmaceutical and Regulatory Affairs Address: 10-16 Grenada Way Kingston 5 Phone: 876-633-8172 Email: |
|
Legal base of the Procedure |
The Foods and Drugs Act |
|
Processing time |
N/A |
|
Fee (Import permit) |
Not Available |
Required Information
|
No. |
Type of information |
Note |
|
1 |
Application Form, supporting documents |
Process Steps
|
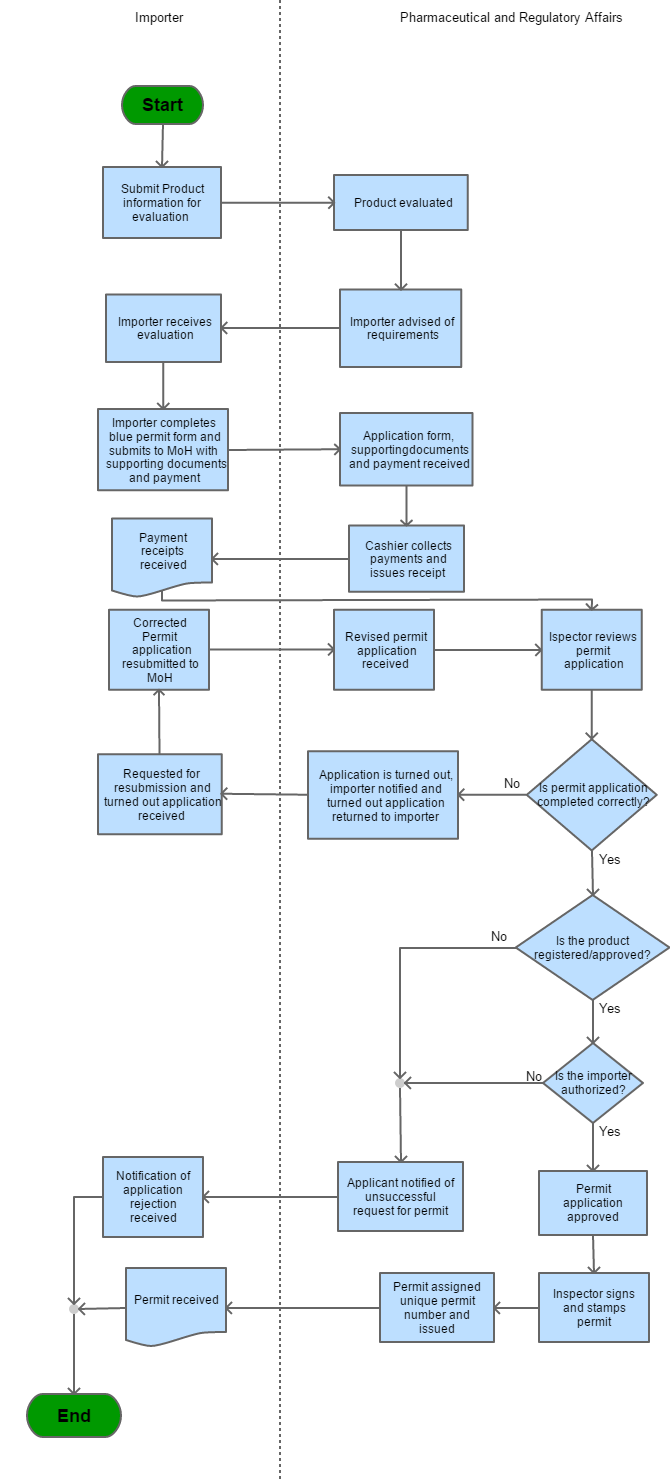
Step 1 |
Submit product information to Pharmaceutical and Regulatory Affairs (PRA) |
|
|
Step 2 |
PRA evaluates information and advises importer of requirements | |
|
Step 3 |
Importer receives evaluation, completes permit application and makes payment |
|
|
Step 4 |
Cashier receives payment and issues receipt to importer. | |
| Step 5 | PRA receives application form, supporting documents and payment | |
| Step 6 | PRA Inspector reviews permit application | |
| Step 7 |
a) If permit application is deemed to be incorrectly completed; i) the application is turned out, the importer is notified and the application returned. ii) The importer then corrects the application and resubmits to MoH, Step 6 is repeated b) If permit application is deemed to be correctly completed, go to step 8. |
|
| Step 8 |
a) If the product is not registered/approved by PRA; the applicant is notified of the unsuccessful request for permit. b) If the product is registered /approved by PRA; the PRA then verifies that the importer is authorized. |
|
| Step 9 |
If the importer has been authorized by the PRA to import; the application is approved. Go to Step 10 If not, the importer is notified of the unsuccessful request for the permit. |
|
| Step 10 | The Inspector signs and stamps the permit | |
| Step 11 | PRA assigns a unique permit number and the permit issued to the importer. |
| # | Title | Description | Issued By | File |
|---|---|---|---|---|
| 1 | Ministry of Health Permit Application for Cosmetics and Chemicals | Ministry of Health Permit Application for Cosmetics and Chemicals | Ministry of Health & Wellness |
| # | Name | Description | Measure Type | Agency | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|---|
| 1 | Importation of Medical Devices and Medicines Cosmetics | The importation of "Medical Devices and Medicines and Cosmetics requires an importer needs to obtain a permit from the Ministry of Health prior to the importation and sale of medical devices, medicines, and comestics in Jamaica. Importers of controlled substances are required under the law- Food and Drug Act 1975 to get a permit | Permit Requirement | Ministry of Health & Wellness | Importers are required to register with the Ministry of Health to receive a permit. Importers need to complete the Pink and Yellow form entitled " Permit Application for Cosmetics and Chemicals" and Permit Application for Psychotropics, Narcotics, and Precursors" respectively | The Food and Drugs Act | 9999-09-09 | Good |
Please share your feedback below and help us improve our content.
