|
Category |
Import Permit |
|
Responsible Agency |
Pharmaceutical and Regulatory Affairs Address: 10-16 Grenada Way Kingston 5 Phone: 876-633-8172 Email: |
|
Legal base of the Procedure |
The Foods and Drugs Act |
Required Information
|
No. |
Type of information |
Note |
|
1 |
Application form, supporting documents |
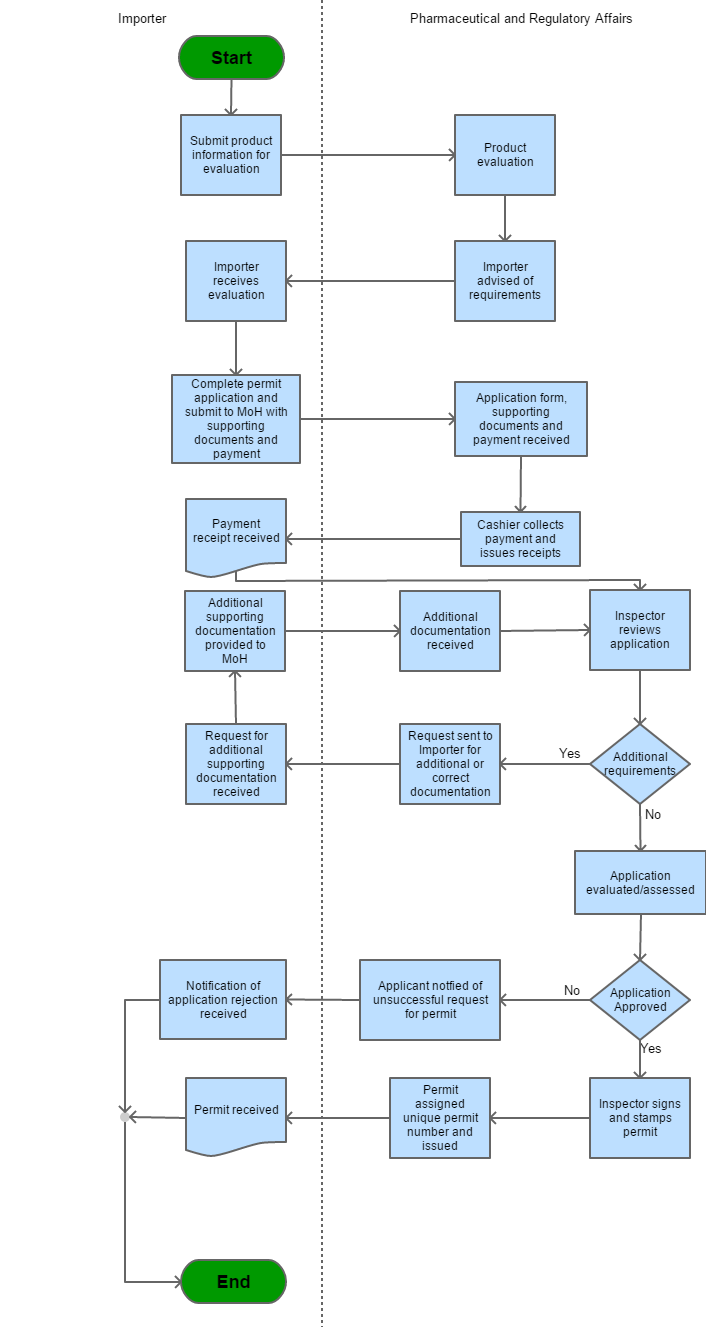
Process Steps
|
Step 1 |
The client wants to import product |
|
|
Step 2 |
The product information is submitted for evaluation | |
|
Step 3 |
Product information is evaluated |
|
|
Step 4 |
The client/ Importer advised of requirements |
|
|
Step 5 |
Client/ Importer receives evaluation |
|
| Step 6 | Importer completes permit application and submits to MOH with supporting documents and payment | |
| Step 7 |
Application form, supporting documents and payment received |
|
| Step 8 |
Cashier collects payment and issues receipt |
|
| Step 9 |
Payment receipt received |
|
| Step 10 | Inspector review application | |
| Step 11 | If additional documents are needed a request is sent to the importer for additional or correct documentation, the additional documents are received. Documents are given to the MOH and the inspector reviews documents. However, if no additional documents are required the application will be evaluated. | |
| Step 12 | If the application is not approved the applicant will be notified that his/ her application was rejected. However, if the application is approved the inspector stamps the permit and assigns a unique permit number | |
| Step 13 | The permit is issued | |
| Step 14 | Permit is received |
| # | Title | Description | Issued By | File |
|---|---|---|---|---|
| 1 | Ministry of Health Permit Application for Food and Drugs | Ministry of Health Permit Application for Food and Drugs | Ministry of Health & Wellness |
| # | Name | Description | Measure Type | Agency | Comments | Legal Document | Validity To | Measure Class |
|---|---|---|---|---|---|---|---|---|
| 1 | Pharmaceutical Chemical and Food Additives Import Permit | A person shall not import any drugs unless (s)he has applied for and obtained a permit from the Ministry of Health and has paid a fee of $200. A single permit is only applicable to a maximum of ten (10) products. | Permit Requirement | Ministry of Health & Wellness | A person shall not sell, manufacture, import or distribute a drug unless- (a) that drug has been registered with the Ministry of Health; and (b) a fee of $25.00 has been paid in respect of such registration. Drugs must be appropriately labelled. | The Food and Drug Regulations 1975 | 9999-09-09 | Good |
Please share your feedback below and help us improve our content.
